* In the formation of Dinitrogen molecule, each nitrogen atom contributes 3 Figure 7.12 shows the Lewis structures for two hypervalent molecules, PCl5 and SF6. Question to ponder: What is the overall polarity of the carbon dioxide 12.4: Covalent Bonds and Lewis Structures, [ Complete the octets around the surrounding atoms (except for H). * The ...In this interactive and animated object, students distribute the valence electrons in simple covalent molecules with one central atom. Six rules are followed to show the bonding and nonbonding electrons in Lewis dot structures. The process is well illustrated with eight worked examples and two interactive practice problems.
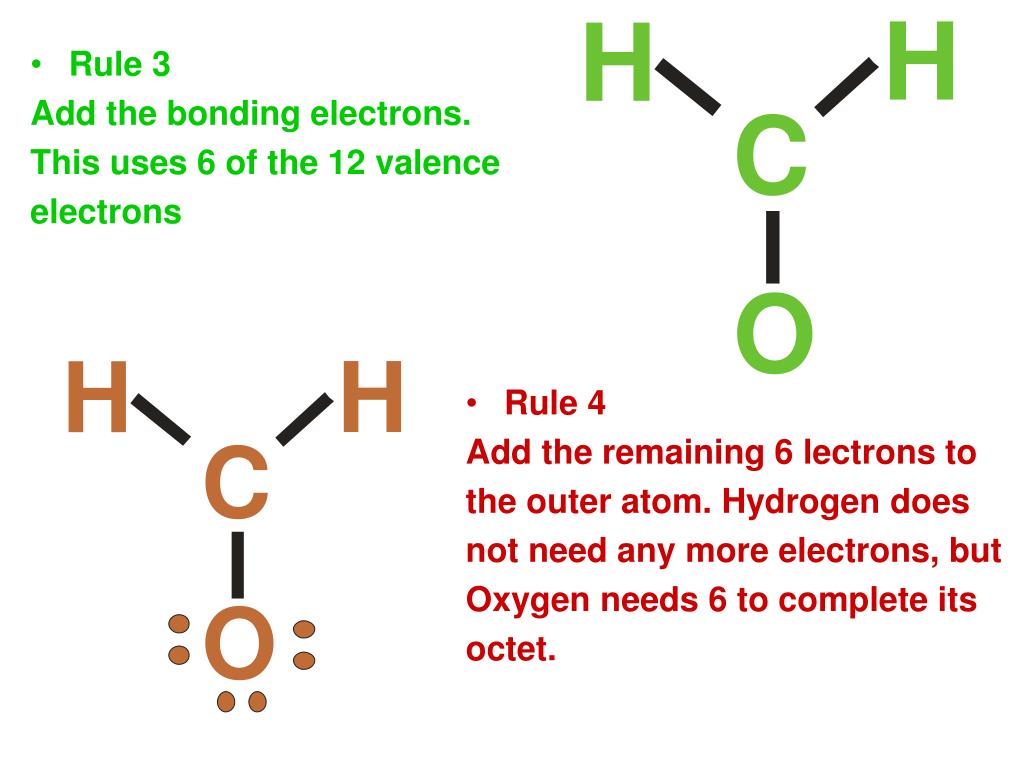

Covalent Lewis Dot Structure
. (3c) Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. Start studying Molecular Geometry - Covalent Bonding, Lewis Dot Structures, VSEPR, Quantum Numbers, Electron Configuration, and Orbitals. Notice from the preceding example that the Lewis diagrams of the alkali metals are identical except for their chemical symbols.


