- Lewis Dot Structure Bond Calculator Free
- Lewis Dot Structure Practice Worksheet
- Lewis Dot Structure Bond Calculator Worksheet
- Lewis Dot Structure Calc
- Lewis Dot Structure Finder
What is a Lewis Diagram?
Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom. For example, the Lewis diagrams for hydrogen, helium, and carbon areNov 23, 2011 Is it possible to make a Lewis structure for metallic bonds.? For our project on bonds, the teacher wants us to draw at least two examples of each bonds, including metallic bonds. I have searched everywhere on how to do this, but there is not an answer anywhere. There are three basic steps to determining the bond angles in a molecule: 1. Write the Lewis dot structure for the molecule. Assume that you must determine the bond angles in 'BF'3. 'B' is less electronegative than 'F', so 'B' becomes the central atom. If we have three 'F' atoms, that means that we are going to use all three electrons from the 'B'. This gives us three bonding pairs of. Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (Lewis structures). Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired with one of the first set of dots, with a maximum.
where the symbol represents the element (in this case, hydrogen, helium, and carbon) and the dots represent the electrons in the outer shell (in this case, one, two, and four). These diagrams are based on the electron structures learned in the Atomic Structure and Periodic Table chapters.
What is a Lewis Structure?
The Lewis structure is used to represent the covalent bonding of a molecule or ion. Covalent bonds are a type of chemical bonding formed by the sharing of electrons in the valence shells of the atoms. Covalent bonds are stronger than the electrostatic interactions of ionic bonds, but keep in mind that we are not considering ionic compounds as we go through this chapter. Most bonding is not purely covalent, but is polar covalent (unequal sharing) based on electronegativity differences.
The atoms in a Lewis structure tend to share electrons so that each atom has eight electrons (the octet rule). The octet rule states that an atom in a molecule will be stable when there are eight electrons in its outer shell (with the exception of hydrogen, in which the outer shell is satisfied with two electrons). Lewis structures display the electrons of the outer shells because these are the ones that participate in making chemical bonds.
How to Build a Lewis Structure?
For simple molecules, the most effective way to get the correct Lewis structure is to write the Lewis diagrams for all the atoms involved in the bonding and adding up the total number of valence electrons that are available for bonding. For example, oxygen has 6 electrons in the outer shell, which are the pattern of two lone pairs and two singles. If the electrons are not placed correctly, one could think that oxygen has three lone pairs (which would not leave any unshared electrons to form chemical bonds). After adding the four unshared electrons around element symbol, form electron pairs using the remaining two outer shell electrons.
| Incorrect Structure | Correct Structure |
 are two hydrogen atoms and one oxygen atom. The Lewis structure of each of these atoms would be as follows:
are two hydrogen atoms and one oxygen atom. The Lewis structure of each of these atoms would be as follows:One good example is the water molecule. Water has the chemical formula of H2O, which means there
We can now see that we have eight valence electrons (six from oxygen and one from each hydrogen). With few exceptions, hydrogen atoms are always placed on the outside of the molecule, and in this case the central atom would be oxygen. Each of the two unpaired electrons of the oxygen atom will form a bond with one of the unpaired electrons of the hydrogen atoms. The bonds formed by the shared electron pairs can be represented by either two closely places dots between two element symbols or more commonly by a straight line between element symbols:
Let us try another one.
Example: Write the Lewis structure for methane (CH4).
Answer:Hydrogen atoms are always placed on the outside of the molecule, so carbon should be the central atom.
After counting the valence electrons, we have a total of 8 [4 from carbon + 4(1 from each hydrogen] = 8.
Each hydrogen atom will be bonded to the carbon atom, using two electrons. The four bonds represent the eight valence electrons with all octets satisfied, so your structure is complete.
Example: Write the Lewis structure for carbon dioxide (CO2).
Answer:Carbon is the lesser electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 16 [4 from carbon + 2(6 from each oxygen)] = 16.
Each oxygen atom has two unshared electrons that can be used to form a bond with two unshared electrons of the carbon atom, forming a double bond between the two atoms. The remaining eight electrons will be place on the oxygen atoms, with two lone pairs on each.
Lewis Structures of Polyatomic Ions
Building the Lewis Structure for a polyatomic ion can be done in the same way as with other simple molecules, but we have to consider that we will need to adjust the total number of electrons for the charge on the polyatomic ion. If the ion has a negative charge, the number of electrons that is equal to the charge on the ion should be added to the total number of valence electrons. If the ion has a positive charge, the number of electrons that is equal to the charge should be subtracted from the total number of valence electrons. After writing the structure, the entire structure should then be placed in brackets with the charge on the outside of the brackets at the upper right corner.
Example: Write the Lewis structure for the ammonium ion (NH4+).
Answer: Hydrogen atoms are always placed on the outside of the molecule, so nitrogen should be the central atom.
After counting the valence electrons, we have a total of 9 [5 from nitrogen + 4(1 from each hydrogen)] = 9. The charge of +1 means an electron should be subtracted, bringing the total electron count to 8.
Each hydrogen atom will be bonded to the nitrogen atom, using two electrons. The four bonds represent the eight valence electrons with all octets satisfied, so your structure is complete. (Do not forget your brackets and to put your charge on the outside of the brackets)
Example: Write the Lewis structure for the hydroxide ion (OH-).
After counting the valence electrons, we have a total of 7 [6 from Oxygen + 1 from each Hydrogen)] = 7. The charge of -1 indicates an extra electron, bringing the total electron count to 8.
Oxygen will be bonded to the hydrogen, using two electrons. Place the remaining six electrons as three lone pairs on the oxygen atom. All octets are satisfied, so your structure is complete. (Do not forget your brackets and to put your charge on the outside of the brackets)
Lewis Structures for Resonance Structures
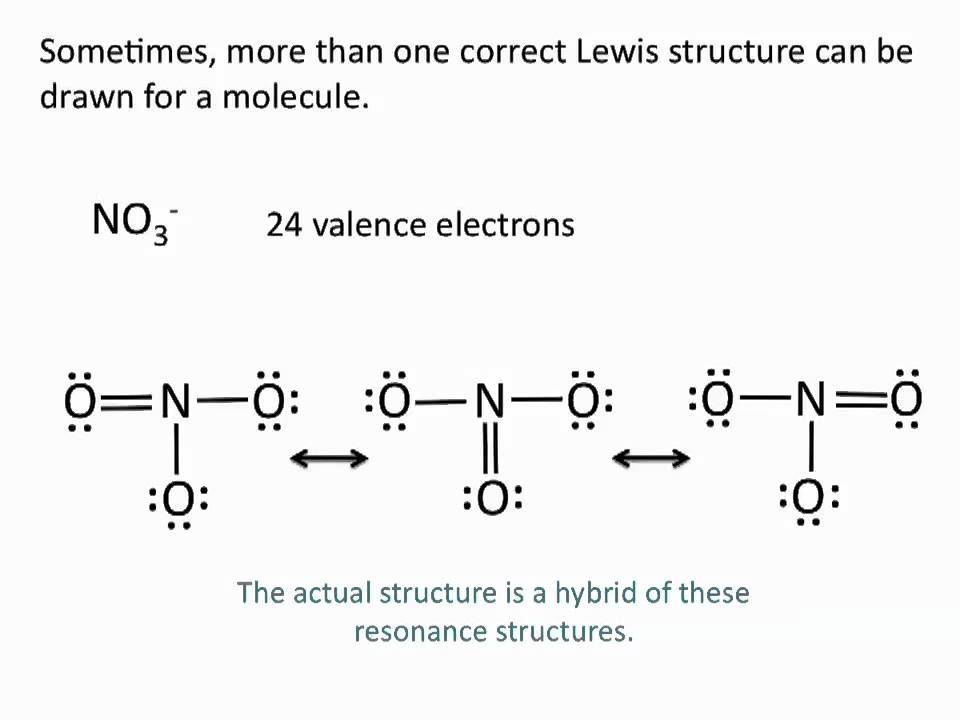
The existence of some molecules often involves two or more structures that are equivalent. Resonance can be shown using Lewis structures to represent the multiple forms that a molecule can exist. The molecule is not switching between these forms, but is rather an average of the multiple forms. This can be seen when multiple atoms of the same type surround the central atom. When all lone pairs are placed on the structure, all the atoms may still not have an octet of electrons. To deal with this problem, the atoms (primarily in a C, N, or O formula) form double or triple bonds by moving lone pairs to form a second or third bond between two atoms. The atom that originally had the lone pair does not lose its octet because it is sharing its lone pair. Double-headed arrows are placed between the multiple structures of the molecule or ion to show resonance. Let us look at how to build a nitrate ion (NO3-).
Nitrogen is the least electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 23[5 from nitrogen + 3(6 from each oxygen)] = 23. The charge of -1 indicates an extra electron, bringing the total electron count to 24.
Each oxygen atom will be bonded to the nitrogen atom, using a total of six electrons. We then place the remaining 18 electrons initially as 9 lone pairs on the oxygen atoms (3 pairs around each atom).
Although all 24 electrons are represented in the structure (two electrons for each of the three bonds and 18 for each of the nine lone pairs), the octet for the nitrogen atom is not satisfied. To satisfy the octet rule for the nitrogen atom, a double bond needs to be made between the nitrogen and one of the oxygen atoms. Because of the symmetry of the molecule, it does not matter which oxygen atoms is chosen. Because there are three different oxygen atoms that could form the double bond, there will be three different resonance structures showing each oxygen atom with a double bond to the nitrogen atom. Double-headed arrows will be placed between these three structures. (Do not forget your brackets and to put your charge on the outside of the brackets)
Example: What is the Lewis structure for the nitrite ion (NO2−)?
Answer: Nitrogen is the least electronegative atom and should be the central atom.
After counting the valence electrons, we have a total of 17 [5 from nitrogen + 2(6 from each oxygen)] = 17. The charge of -1 indicates an extra electron, bringing the total electron count to 18.
Each oxygen will be bonded to the nitrogen, using two electrons. Place the remaining 16 electrons initially as nine lone pairs on the oxygen atoms (3 pairs around each atom) and the nitrogen (one pair).
Although all 18 electrons are represented in the structure (2 electrons for each of the two bonds and 14 for each of the seven lone pairs), the octet for the nitrogen atom is not satisfied. To satisfy the octet rule for the nitrogen atom, a double bond needs to be made between the nitrogen atom and one of the oxygen atoms. Because of the symmetry of the molecule, it does not matter which oxygen is chosen. Because there are two different oxygen atoms that could form the double bond, there will be two different resonance structures showing each oxygen atom with a double bond to the nitrogen atom. A double-headed arrow will be placed between these structures. (Do not forget your brackets and to put your charge on the outside of the brackets)
Lewis Structures for Electron-rich Compounds
Elements with atomic number greater than 13 often form compounds or polyatomic ions in which there are “extra” electrons. For these compounds we proceed as above. Once all of the octets are satisfied, the extra electrons are assigned to the central atom either as lone pairs or an increase in the number of bonds. (Never use multiple bonds with these compounds—you already have too many electrons.)Example: Draw the Lewis structure for phosphorus pentafluoride, PF5.
Answer:The electronegativity of fluorine is greater than that of phosphorus—so the phosphorus atom is placed in the center of the molecule.The total number of electros is 40 [5(7 from each fluorine) + 5 from the phosphorus] = 40. Using a single bond between the phosphorus atom and each of the fluorine atoms and filling the remaining electrons to satisfy the octet rule for the fluorine atoms accounts for all 40 electrons. Note that there are five bonds around the central atom.
Lewis Structures for Electron-poor Compounds
There is another type of molecule or polyatomic ion in which there is an electron deficiency of one or more electrons needed to satisfy the octets of all the atoms. In these cases, the more electronegative atoms are assigned as many electrons to complete those octets first and then the deficiency is assigned to the central atom.Example: Draw the Lewis structure for boron trifluoride, BF3.
Answer:The electronegativity of fluorine is greater than that of boron—so the boron atom is placed in the center of the molecule.The total number of electron is 24 [3(7 from each fluorine) + 3 from boron] = 24. Using a single bond between the boron and each of the fluorine atoms and filling the remaining electron as lone pairs around the fluorine atoms to satisfy the octets accounts for all 24 electrons.
The boron atom is two electrons shy of its octet. You may ask about the formation of a double bond (and even resonance). But, fluorine and boron are not in the list that can form double bonds (C, N, O, P, S) and so the compound is electron poor.
Try It Out!
Draw the Lewis structure for the following:
- Hydronium ion (H3O+)
- Hypochlorite ion (ClO-)
- Carbonate ion (CO3-2)
- Ammonia (NH3)
- Hydrogen fluoride (HF)
- Ozone (O3)
- Xenon difluoride (XeF2)
Representing Valence Electrons in Lewis Symbols
Lewis symbols use dots to visually represent the valence electrons of an atom.
Learning Objectives
Recall the Lewis structure formalism for representing valance electrons
Key Takeaways
Key Points
- Electrons exist outside of an atom ‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
- The outermost principal energy level that contains electrons is called the valence level and contains valence electrons.
- Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs.
- Lewis symbols do not visualize the electrons in the inner principal energy levels.
Key Terms
- principal energy levels: The different levels where electrons can be found and that occur at specific distances from the atom’s nucleus. Each level is associated with a particular energy value that electrons within it have.
- valence level: The outermost principal energy level, which is the level furthest away from the nucleus that still contains electrons.
- valence electrons: The electrons of atoms that participate in the formation of chemical bonds.
- Lewis symbols: Symbols of the elements with their number of valence electrons represented as dots
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are diagrams that represent the valence electrons of an atom. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
Principal Energy Levels
An atom consists of a positively charged nucleus and negatively charged electrons. The electrostatic attraction between them keeps electrons ‘bound’ to the nucleus so they stay within a certain distance of it. Careful investigations have shown that not all electrons within an atom have the same average position or energy. We say the electrons ‘reside’ in different principal energy levels, and these levels exist at different radii from the nucleus and have rules regarding how many electrons they can accommodate.
Principal energy levels of gold (Au): The figure shows the organization of the electrons around the nucleus of a gold (Au) atom. Notice that the first energy level (closest to the nucleus) can have only two electrons, while more electrons can ‘fit’ within a given level further out. The number of electrons in each level is listed on the upper right corner of the figure. Notice that the outermost level has only one electron.
As an example, a neutral atom of gold (Au) contains 79 protons in its nucleus and 79 electrons. The first principal energy level, which is the one closest to the nucleus, can hold a maximum of two electrons. The second principal energy level can have 8, the third can have 18, and so on, until all 79 electrons have been distributed.
The outermost principal energy level is of great interest in chemistry because the electrons it holds are the furthest away from the nucleus, and therefore are the ones most loosely held by its attractive force; the larger the distance between two charged objects, the smaller the force they exert on each other. Chemical reactivity of all of the different elements in the periodic table depends on the number of electrons in that last, outermost level, called the valence level or valence shell. In the case of gold, there is only one valence electron in its valence level.
Octet of Valence Electrons
Atoms gain, lose, or share electrons in their valence level in order to achieve greater stability, or a lower energy state. From this perspective, bonds between atoms form so that the bonded atoms are in a lower energy state compared to when they were by themselves. Atoms can achieve this more stable state by having a valence level which contains as many electrons as it can hold. For the first principal energy level, having two electrons in it is the most stable arrangement, while for all other levels outside of the first, eight electrons are necessary to achieve the most stable state.
Lewis Symbols
In the Lewis symbol for an atom, the chemical symbol of the element (as found on the periodic table) is written, and the valence electrons are represented as dots surrounding it. Only the electrons in the valence level are shown using this notation. For example, the Lewis symbol of carbon depicts a “C’ surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2.
The Lewis symbol for carbon: Each of the four valence electrons is represented as a dot.
Electrons that are not in the valence level are not shown in the Lewis symbol. The reason for this is that the chemical reactivity of an atom of the element is solely determined by the number of its valence electrons, and not its inner electrons. Lewis symbols for atoms are combined to write Lewis structures for compounds or molecules with bonds between atoms.
Writing Lewis Symbols for Atoms
The Lewis symbol for an atom depicts its valence electrons as dots around the symbol for the element.
Key Takeaways
Key Points
- The columns, or groups, in the periodic table are used to determine the number of valence electrons for each element.
- The noble/ inert gases are chemically stable and have a full valence level of electrons.
- Other elements react in order to achieve the same stability as the noble gases.
- Lewis symbols represent the valence electrons as dots surrounding the elemental symbol for the atom.
Key Terms
- group: A column in the periodic table that consists of elements with similar chemical reactivity, because they have the same number of valence electrons.
- Noble Gases: Inert, or unreactive, elements in the last group in the periodic table which are typically found in the gaseous form.
- Lewis symbol: Formalism in which the valence electrons of an atom are represented as dots.
Determining the Number of Valence Electrons
In order to write the Lewis symbol for an atom, you must first determine the number of valence electrons for that element. The arrangement of the periodic table can help you figure out this information. Since we have established that the number of valence electrons determines the chemical reactivity of an element, the table orders the elements by number of valence electrons.
Each column (or group) of the periodic table contains elements that have the same number of valence electrons. Furthermore, the number of columns (or groups) from the left edge of the table tells us the exact number of valence electrons for that element. Recall that any valence level can have up to eight electrons, except for the first principal energy level, which can only have two.
Periodic table of the elements: Group numbers shown by Roman numerals (above the table) tell us how many valence electrons there are for each element.
Some periodic tables list the group numbers in Arabic numbers instead of Roman numerals. In that case, the transition metal groups are included in the counting and the groups indicated at the top of the periodic table have numbers 1, 2, 13, 14, 15, 16, 17, 18. The corresponding roman numerals used are I, II, III, IV, V, VI, VII, VIII.
Survey of the Groups in the Periodic Table
Take the first column or group of the periodic table (labeled ‘I’): hydrogen (H), lithium (Li), sodium (Na), potassium (K), etc. Each of these elements has one valence electron. The second column or group (labeled ‘II’) means that beryllium (Be), magnesium (Mg), calcium (Ca), etc., all have two valence electrons.
The middle part of the periodic table that contains the transition metals is skipped in this process for reasons having to do with the electronic configuration of these elements.
Proceeding to the column labeled ‘III’, we find that those elements (B, Al, Ga, In,…) have three valence electrons in their outermost or valence level.
We can continue this inspection of the groups until we reach the eighth and final column, in which the most stable elements are listed. These are all gaseous under normal conditions of temperature and pressure, and are called ‘noble gases.’ Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their valence level. Therefore, these elements have a full valence level that has the maximum number of electrons possible. Helium (He), at the very top of this column is an exception because it has two valence electrons; its valence level is the first principal energy level which can only have two electrons, so it has the maximum number of electrons in its valence level as well.
The Lewis symbol for helium: Helium is one of the noble gases and contains a full valence shell. Unlike the other noble gases in Group 8, Helium only contains two valence electrons. In the Lewis symbol, the electrons are depicted as two lone pair dots.

The noble gases represent elements of such stability that they are not chemically reactive, so they can be called inert. In other words, they don’t need to bond with any other elements in order to attain a lower energy configuration. We explain this phenomenon by attributing their stability to having a ‘full’ valence level.
The significance in understanding the nature of the stability of noble gases is that it guides us in predicting how other elements will react in order to achieve the same electronic configuration as the noble gases by having a full valence level.
Writing Lewis Symbols for Atoms
Lewis symbols for the elements depict the number of valence electrons as dots. In accordance with what we discussed above, here are the Lewis symbols for the first twenty elements in the periodic table. The heavier elements will follow the same trends depending on their group.

Once you can draw a Lewis symbol for an atom, you can use the knowledge of Lewis symbols to create Lewis structures for molecules.
Valence Electrons and the Periodic Table: Electrons can inhabit a number of energy shells. Different shells are different distances from the nucleus. The electrons in the outermost electron shell are called valence electrons, and are responsible for many of the chemical properties of an atom. This video will look at how to find the number of valence electrons in an atom depending on its column in the periodic table.
Introduction to Lewis Structures for Covalent Molecules
In covalent molecules, atoms share pairs of electrons in order to achieve a full valence level.
Learning Objectives
Predict and draw the Lewis structure of simple covalent molecules and compounds
Key Takeaways
Key Points
- The octet rule says that the noble gas electronic configuration is a particularly favorable one that can be achieved through formation of electron pair bonds between atoms.
- In many atoms, not all of the electron pairs comprising the octet are shared between atoms. These unshared, non-bonding electrons are called ‘ lone pairs ‘ of electrons.
- Although lone pairs are not directly involved in bond formation, they should always be shown in Lewis structures.
- There is a logical procedure that can be followed to draw the Lewis structure of a molecule or compound.
Key Terms
- octet rule: Atoms try to achieve the electronic configuration of the noble gas nearest to them in the periodic table by achieving a full valence level with eight electrons.
- exceptions to the octet rule: Hydrogen (H) and helium (He) only need two electrons to have a full valence level.
- covalent bond: Two atoms share valence electrons in order to achieve a noble gas electronic configuration.
- Lewis structure: Formalism used to show the structure of a molecule or compound, in which shared electrons pairs between atoms are indicated by dashes. Non-bonding, lone pairs of electrons must also be shown.
The Octet Rule
Noble gases like He, Ne, Ar, Kr, etc., are stable because their valence level is filled with as many electrons as possible. Eight electrons fill the valence level for all noble gases, except helium, which has two electrons in its full valence level. Other elements in the periodic table react to form bonds in which valence electrons are exchanged or shared in order to achieve a valence level which is filled, just like in the noble gases. We refer to this chemical tendency of atoms as ‘the octet rule,’ and it guides us in predicting how atoms combine to form molecules and compounds.
Covalent Bonds and Lewis Diagrams of Simple Molecules
The simplest example to consider is hydrogen (H), which is the smallest element in the periodic table with one proton and one electron. Hydrogen can become stable if it achieves a full valence level like the noble gas that is closest to it in the periodic table, helium (He). These are exceptions to the octet rule because they only require 2 electrons to have a full valence level.
Two H atoms can come together and share each of their electrons to create a ‘ covalent bond.’ The shared pair of electrons can be thought of as belonging to either atom, and thus each atom now has two electrons in its valence level, like He. The molecule that results is H2, and it is the most abundant molecule in the universe.
Lewis Dot Structure Bond Calculator Free
Lewis structure of diatomic hydrogen: This is the process through which the H2 molecule is formed. Two H atoms, each contributing an electron, share a pair of electrons. This is known as a ‘single covalent bond.’ Notice how the two electrons can be found in a region of space between the two atomic nuclei.
The Lewis formalism used for the H2 molecule is H:H or H—H. The former, known as a ‘Lewis dot diagram,’ indicates a pair of shared electrons between the atomic symbols, while the latter, known as a ‘Lewis structure,’ uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. The electrons are color-coded to indicate which atoms they belonged to before the covalent bonds formed, with red representing hydrogen and blue representing carbon. Four covalent bonds are formed so that C has an octet of valence electrons, and each H has two valence electrons—one from the carbon atom and one from one of the hydrogen atoms.
Now consider the case of fluorine (F), which is found in group VII (or 17) of the periodic table. It therefore has 7 valence electrons and only needs 1 more in order to have an octet. One way that this can happen is if two F atoms make a bond, in which each atom provides one electron that can be shared between the two atoms. The resulting molecule that is formed is F2, and its Lewis structure is F—F.
Achieving an octet of valence electrons: Two fluorine atoms are able to share an electron pair, which becomes a covalent bond. Notice that only the outer (valence level) electrons are involved, and that in each F atom, 6 valence electrons do not participate in bonding. These are ‘lone pairs’ of electrons.
After a bond has formed, each F atom has 6 electrons in its valence level which are not used to form a bond. These non-bonding valence electrons are called ‘lone pairs’ of electrons and should always be indicated in Lewis diagrams.
Lewis structure of acetic acid: Acetic acid, CH3COOH, can be written out with dots indicating the shared electrons, or, preferably, with dashes representing covalent bonds. Notice the lone pairs of electrons on the oxygen atoms are still shown. The methyl group carbon atom has six valence electrons from its bonds to the hydrogen atoms because carbon is more electronegative than hydrogen. Also, one electron is gained from its bond with the other carbon atom because the electron pair in the C−C bond is split equally.
Lewis Dot Structure Practice Worksheet
Procedure for Drawing Simple Lewis Structures
We have looked at how to determine Lewis structures for simple molecules. The procedure is as follows:
- Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the most number of possible bonds to be the central atom).
- Draw Lewis symbols of the individual atoms in the molecule.
- Bring the atoms together in a way that places eight electrons around each atom (or two electrons for H, hydrogen) wherever possible.
- Each pair of shared electrons is a covalent bond which can be represented by a dash.
Lewis Dot Structure Bond Calculator Worksheet
Alternate view of lewis dot structure of water: This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons, and each H atom having two valence electrons.
Multiple bonds can also form between elements when two or three pairs of electrons are shared to produce double or triple bonds, respectively. The Lewis structure for carbon dioxide, CO2, is a good example of this.
Lewis structure of carbon dioxide: This figure explains the bonding in a CO2 molecule. Each O atom starts out with six (red) electrons and C with four (black) electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C.
In order to achieve an octet for all three atoms in CO2, two pairs of electrons must be shared between the carbon and each oxygen. Since four electrons are involved in each bond, a double covalent bond is formed. You can see that this is how the octet rule is satisfied for all atoms in this case. When a double bond is formed, you still need to show all electrons, so double dashes between the atoms show that four electrons are shared.
Final Lewis structure for carbon dioxide: Covalent bonds are indicated as dashes and lone pairs of electrons are shown as pairs of dots. in carbon dioxide, each oxygen atom has two lone pairs of electrons remaining; the covalent bonds between the oxygen and carbon atoms each use two electrons from the oxygen atom and two from the carbon.
Lewis Structures for Polyatomic Ions
The Lewis structure of an ion is placed in brackets and its charge is written as a superscript outside of the brackets, on the upper right.
Learning Objectives
Apply the rules for drawing Lewis structures to polyatomic ions
Key Takeaways
Key Points
Lewis Dot Structure Calc
- Ions are treated almost the same way as a molecule with no charge. However, the number of electrons must be adjusted to account for the net electric charge of the ion.
- When counting electrons, negative ions should have extra electrons placed in their Lewis structures, while positive ions should have fewer electrons than an uncharged molecule.
Key Terms
Lewis Dot Structure Finder
- polyatomic ion: A charged species composed of two or more atoms covalently bonded, or of a metal complex that acts as a single unit in acid-base chemistry or in the formation of salts. Also known as a molecular ion.
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons in each individual atom. Non-valence electrons are not represented in Lewis structures. After the total number of available electrons has been determined, electrons must be placed into the structure.
Lewis structures for polyatomic ions are drawn by the same methods that we have already learned. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium ion, NH4+, which contains 9 (5 from N and 1 from each of the four H atoms) –1 = 8 electrons. One electron is subtracted because the entire molecule has a +1 charge.
Coordinate covalent bonding: The ammonium ion, NH4+, contains 9–1 = 8 electrons.
Negative ions follow the same procedure. The chlorite ion, ClO2–, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = 20 electrons. One electron is added because the entire molecule has a -1 charge.
Hypochlorite ion Lewis structure: The hypochlorite ion, ClO−, contains 13 + 1 = 14 electrons.